In vivo performance of engineered implants which determines the device efficiency strongly depends on characteristics of the biomaterials. Engineers contemplating the design of new devices require preliminary information related to the surface characteristics, biocompatibility, and the stability / bioactivity of the materials. These critical aspects raise new concerns in the development of our projects and launch three important research axis. Our goal is to provide data on the health benefits versus the risks of using implant devices and to improve our understanding of how these devices perform clinically.
Our research is focused on the phenomena that occur at the material-tissue interface and aims to determine how those interactions influence the success of implant devices. Research efforts are directed to three specific areas:
- Surface characterization, corrosion and cytotoxicity studies.
- Surface modifications.
- Implant mechanical effects on cell development.
We employ advanced techniques to characterize surfaces, and evaluate biomaterials and implants. Concerning metal alloys, information has been correlated to corrosion and degradation processes, and to the effects of released corrosion products on cell viability and functions. Research has led to the development of a novel cell culture-corrosion system, which permits the simultaneous measurement of corrosion rate and cellular response. We also investigate non-conventional surface modification methods designed to promote the bonding between implants and adjacent tissue, in an effort to determine optimal coating characteristics and promote long term bonding/integration of devices into bony tissues. We investigate how mechanical forces around an implant affect development and maintenance of local tissues and matrix.
Based on the principles of nanoscience and nanotechnology, we are developing new investigation approaches. The application of Nanoscience and Nanotechnology principles can be considered in two ways: on the one hand, it can be viewed as objects with a nano-meter size (a thousandth of a millionth of a meter). And on the other hand, it implies surface modifications and characterization at the nano-scale. We are working on both aspects.
Nanoscale characterization and analysis requires computational techniques for analyzing and designing Microsystems, as well as a knowledge of the basic properties of nanoscale structures and biomolecular conformations. Nanomechanical probes exploit scanning probe techniques for the characterization of physical properties at the nanoscale. Other related techniques permit the characterization of chemical elements. A basic characteristic of nanoscience, that sets it apart from more traditional research areas, is its inherently interdisciplinary nature. While its small scale opens up new possibilities in many individual disciplines, the strongest impact of nanoscience is expected to come from activities at the interface between traditional fields. The realization of our objectives requires much more than exploring small domains with new tools; it will go beyond conventional thinking confined to traditional disciplines. By exploiting the interface among the chemical, physical and biological sciences, we provide complementary data for the construction of devices through the use of nanotechnology.
Nanotechnology aims to construct objects out of their most basic components – in sharp contrast to the typical industrial method of cutting, shaping and assembling products out of bulk materials. The term “nanotechnology,” derived from the Greek word nanos, or “dwarf,” generally refers to engineering and manufacturing at the molecular or nanometer length scale. Building objects molecule by molecule will offer an unprecedented degree of precision and control over the final product. Hence, by using building blocks of molecular scale, devices of great complexity, having thousands or even millions of different mechanical parts, could be fabricated at the microscopic scale. Using these new approaches, we are preparing to promote device miniaturization for nanomedecine. These devices will generate a wide range of new biotechnological enhancements; they are the key to a number of major realizations in medical technology such as the development of artificial organs that mimic the function of real organs more accurately. The following list indicates some of our projects involving surface characterization:
- Morphology and physicochemical characterization of β-TCP samples.
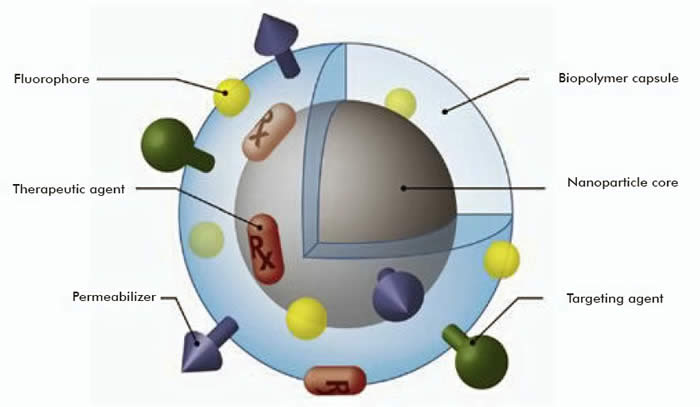
- The application of nanoscale physicochemical analyses to improve the biocompatibility of alginate-based microcapsules used for the transplantation of islet cells.
- Plasma treatment for surface modifications at nanoscale level.
- Biofilm and nanometric characteristics of material surface.
The biocompatibility issue is at the heart of most of our projects because it concerns all categories of materials that we use. Insights obtained on the biocompatibility of materials at the nanoscale are mainly exploited, in our laboratory, in the cardiovascular field.
The biocompatibility of an artificial material in the body is extremely complicated, involving processes traditionally belonging to medical science, surface science, materials science, and molecular biotechnology. Within a few milliseconds after the implant is inserted into the body, a biolayer, consisting of water, proteins and other biomolecules from the physiological liquid, is formed on the implant surface. Subsequently, cells from the surrounding tissue migrate to the area around the implant due to stimulation by cytokines and growth factors within the biolayer. The interaction between an implant surface and the cells is, thus, mediated through this biolayer. The chemical and topographical features of the implant surface strongly influence the properties of the layer and this influence must be understood and controlled, in order to optimize biocompatibility. Since proteins and cells range in size from nano- to micrometers, it is crucial to study materials properties at these length scales. It is well known that most biological phenomena begin at the nanoscale, and these interactions control the mechanisms that govern the response of the tissue to the implant.
Nano-materials such as carbon nanotubes, nanowires, nucleic acids and nano-polypeptides have enormous potential in clinical medicine Therefore, the biocompatibility of nano-materials is of great interest. We are particularly interested in intrinsic issues, such as the structure and function of nanomaterial systems, as well as in the environmental safety of nanoscale materials. A major challenge in the clinical application of nanoscale materials is in determining whether the immune system of the human body can recognize these materials. A special risk related to the possible toxicity and health hazards of nanomaterials is an important factor influencing the success of nanotechnology in medicine. At present, we know very little about the interaction between nano-materials and immune cells. A main aim of our studies is to understand the interactions between these nanomaterials and the immune system.
The electrochemical processing of advanced materials uses knowledge from the fields of electrochemistry, chemical engineering and materials science to develop new materials and processes. The aim is to integrate electrochemical approaches, surface technology and engineering to innovate new products and processes based materials. Currently, we are involved in the development of various aspects of biomaterials based on electrochemical methods:
- The determination of the stability of the biomaterial stability involved in medical devices such as the mechanical heart valve and the pacemaker.
- Modification of the biomaterial surfaces to create suitable interaction between the medical device and its host. In particular the modification works are the following metals and metal alloys used nowadays the medical devices e.g. include β-titanium, and nickel-titanium (Nitinol), alloys exhibiting giant magnetic resistance (GMR).
- The development of significant knowledge on NiTi surface electrochemistry. Accordingly, our Laboratory has made significant contributions on the development of a better understanding of the NiTi surface electrochemistry. We have recently shown that a passive surface layer, which is comprised of titanium oxide approximately 20 nm thick, can be induced to form on the NiTi surface. This layer was found to act as a barrier to prevent the electrochemical corrosion of NiTi. Without the knowledge of the electrochemistry of its surface, nitinol would not be an FDA-approved biocompatible metal, and a whole generation of medical devices would not have developed.
- The development of correlations between the nitinol surface composition and its electrochemical properties. This will help to understand the relation between biomaterial and electrochemical surface properties.
- The development of nano-composite coatings against corrosion, and the self-assembly of biological layers using electrochemistry.


