This research theme investigates imaging beyond diagnosis or guidance purposes, by focusing on the immerging trend which consists in utilizing non-ionizing imaging and real-time feedback to monitor the effect and success of a particular therapeutic procedure. The objective here is to pursue some of our previous work based on CEUS perfusion models and ablation thermometry to allow image-based assessment of a treatment response, so as to monitor capabilities based on ultrasound wave tissue response and multimodal image fusion.
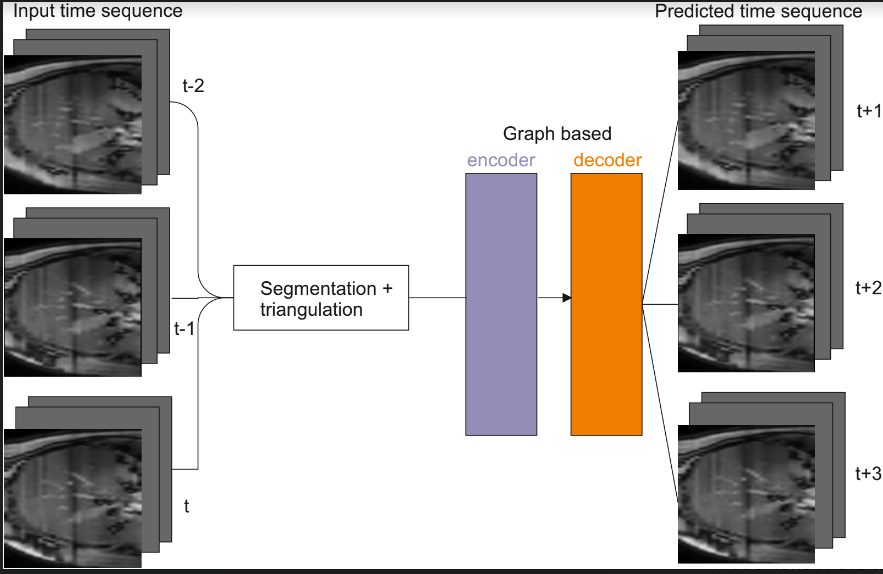
Prediction of Liver motion using recent advances in Temporal Graph Neural Networks
Winter 2022: Matej Gazda (PhD)
Radiation therapy is a well-established treatment to treat malignancies in the thoracic and abdominal regions. However, organs like lungs, liver, or kidneys are subject to respiratory motion, which has a dosimetric impact that decreases the effectiveness of the treatment. This project aims to predict the movement of the tumors and organs under observation, given an input temporal sequence of volumes. First, the organ under observation is represented as a graph by leveraging surface triangulation. Then, the temporal graph is fed into a temporal graph convolutional network that estimates the future movement.
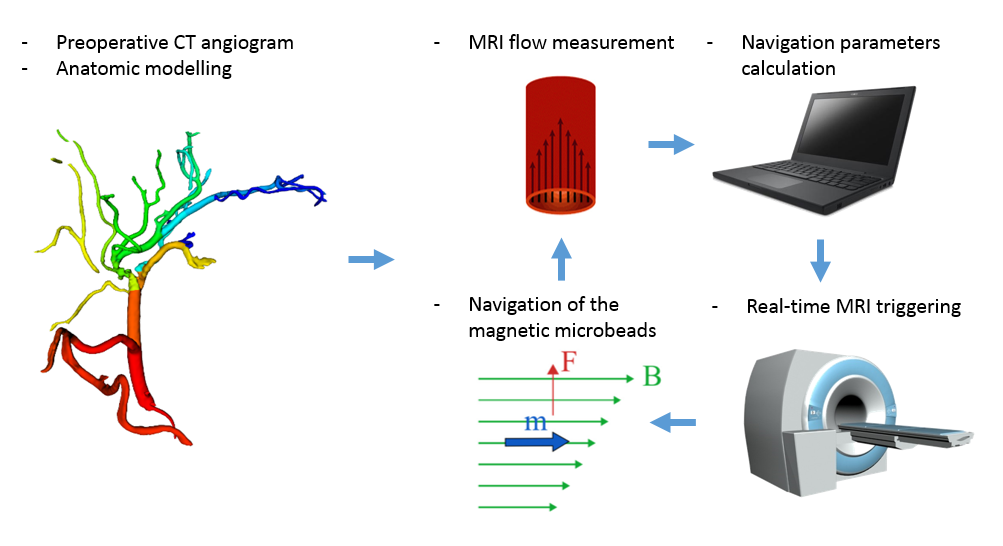
Hepatocellular cancer is the third cause of cancer-related deaths in the world and its incidence is increasing. In the advanced stages of the sickness, transarterial chemoembolization has become the preferred treatment to extend life expectancy. We propose a novel, minimally-invasive treatment that aims to inject biodegradable, drug-carrying, ferromagnetic microbeads in hepatic arteries and navigate them to the tumor using the magnetic field gradients of a clinical MRI machine. This concept, called Magnetic Resonance Navigation (MRN) has shown promising results in vitro as well as in rabbit models, but many challenges remain to be overcome before it can be transferred into clinical practice. One such challenge is to properly define our magnetic gradient parameters from rheological and geometrical data. The imaging phase will allow to gather key rheological and geometrical parameters such as arterial morphology and hepatic blood flow. From this information, the propulsion sequence can be optimized to ensure proper microbead delivery. This part of the project aims at demonstrating from experiments on pig models that blood flow can geometrical features can be extracted from MRI images and used for MRN. We will finally design a platform that integrates 3D artery modeling, real-time rheological parameter monitoring and automated microbeads injection.
Colorectal liver metastasis (CLM) is one of the most aggressive liver malignancies, colorectal cancer (CC) being the 2nd most common cancer with an estimate of 1.36 million cases per year. Due to the difficulty to detect CC in its early stages, there is a very high probability for CLM to develop in almost half of these patients.
The radiologist inability to accurately predict the response to chemotherapy drives the current practice of treating large numbers of patients with CLM knowing only a fraction will benefit, while a significant fraction will endure treatment-related toxicities without benefits.
Hence, there is an urgent need to develop new tools to predict the response of liver metastases to chemotherapy. Monitoring of CLM therapeutic response currently relies on pattern recognition of imaging features and interpretation by radiologists, with poor integration of other data modalities.
We aim to develop and validate treatment outcomes predicting models for CLM using different sources of knowledge like computed tomography (CT), histopathology slides, and clinical information. This will help oncologists monitor CLM therapeutic response to neoadjuvant chemotherapy. Specifically, we aim to:
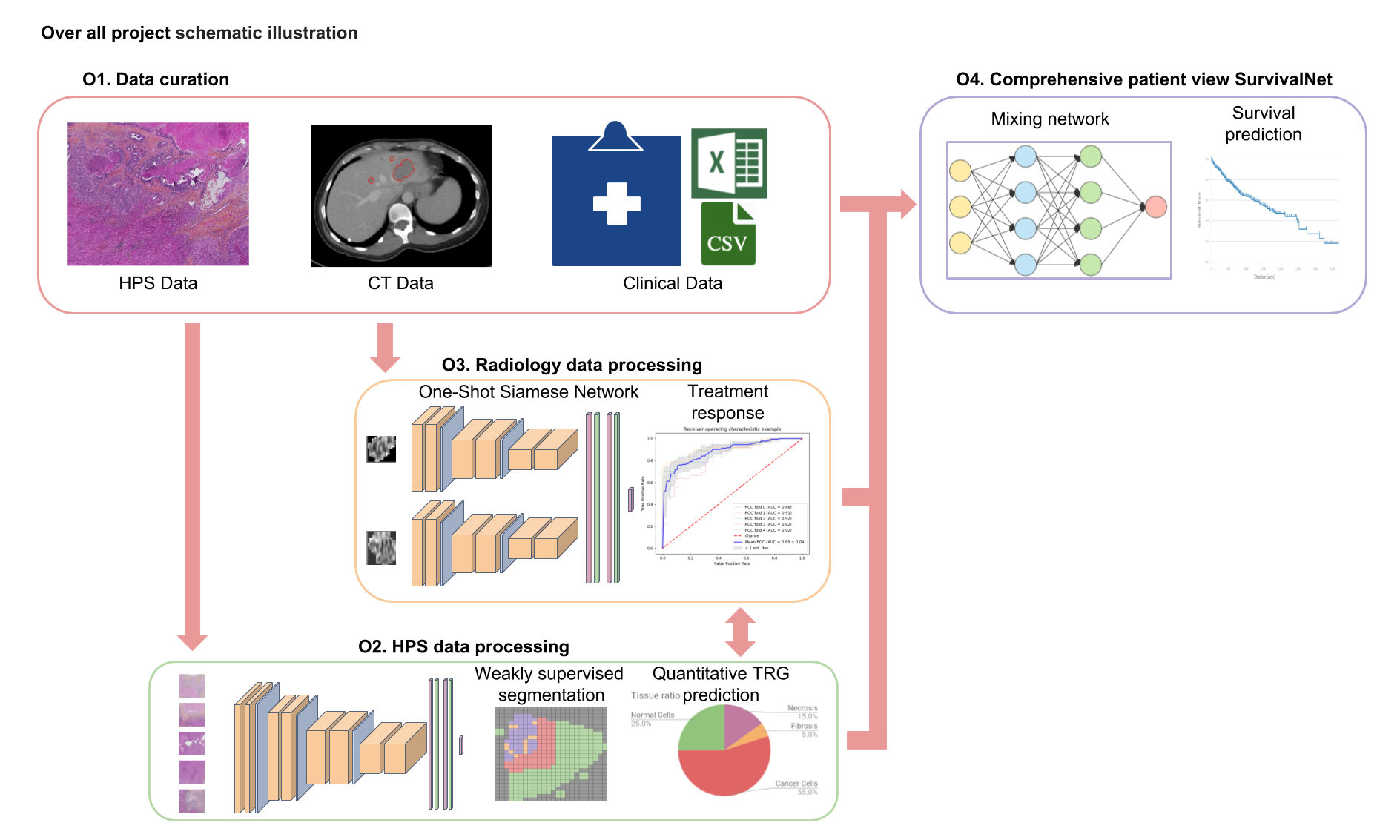
1. Overcome the lack of labels for tissue classification data by the use of semi-supervised learning approach.
2. Improve accuracy for treatment outcome prediction from CT images using one-shot learning.
3. Integrate different medical information sources in an efficient deep learning (DL) scheme that allows better treatment outcome predictions.
Preliminary results show that tissue classification (accuracy > 0.88) using DL techniques is possible. Also, encouraging preliminary results for treatment response from CT images were obtained (accuracy > 0.85).
The economic impact of this project resides on increased productivity on radiologists, oncologist, and pathologist through the use of artificial intelligence tools. The social impact of this project is stated in terms of patient therapy and daycare strategies, at health system level.
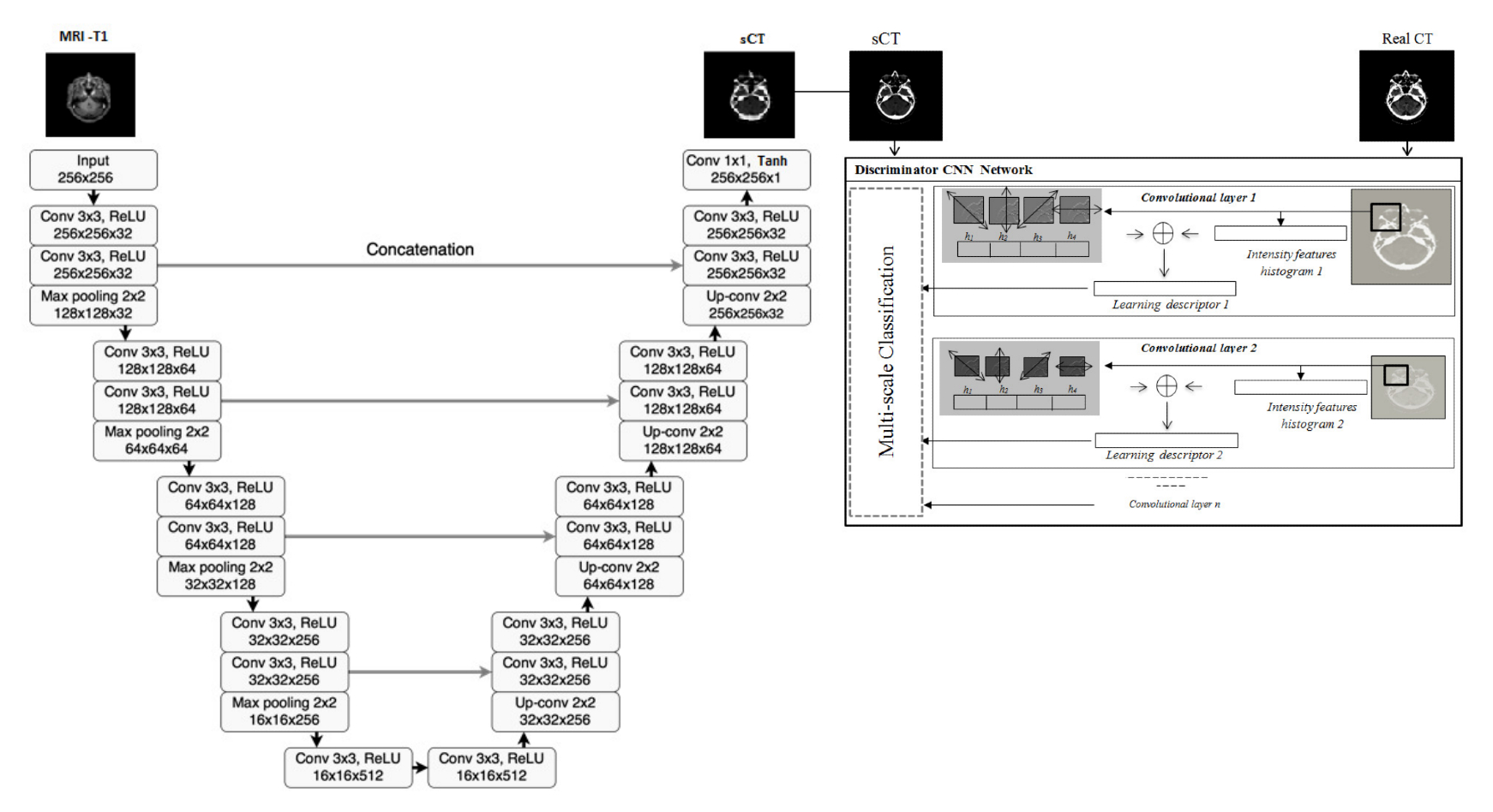
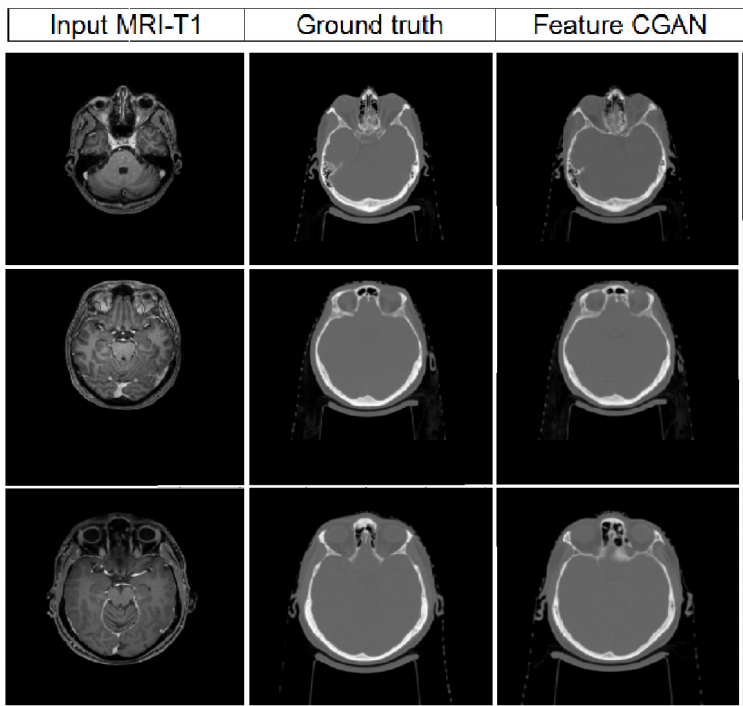
Radiation therapy based MRI is an important part of comprehensive treatment options for curing many cancer indications. One of the major issues, limiting the introduction of MRI into the radiotherapy workflow, is that MRI does not provide information on the electronic density of tissues necessary for dose calculation. The project objective is to design and develop an artificial intelligence-based patient-specific treatment planning system, identifying suitable patients for external beam radiation therapy and adapting to the changing anatomy throughout radiotherapy with an optimal MRI dose plan by balancing normal tissue tolerance and treatment of the tumor. More precisely, the generation of MRI-based dosimetry through a multi-hypotheses pseudo-CT generation with automatic multi-organ segmentation from adaptive multimodal deep learning models. Our goal is to optimize the integration of MRI not only during radiation therapy planning, but also during dose delivery with improved delineation of the target volume and avoidance of critical structures.
Every year, more than 14 million new cases of cancer are diagnosed world-wide, and it is estimated that 50 percent of cancer patients can benefit from external beam radiation therapy (EBRT) to control and manage their disease. In order to avoid damage to healthy tissues around the tumor, the treated organ needs to be constantly imaged and located during treatment. One way to achieve this is by using two-dimensional ultrasound (2D US) to guide the EBRT treatment. However, a real-time three-dimensional (3D) representation of the treated organ, that cannot be easily obtained with 2D US, would improve the effectiveness of EBRT.
Recent advances in machine learning can be used to improve the tracking of organs during 2D US guided EBRT by predicting the 3D shape of the treated organ in real-time during the treatment using readily available 2D US acquisitions and a 3D representation of the organ. The global objective of this project is to develop a platform to monitor and predict the 3D shape of an organ of interest during a medical intervention.
Four-dimensional magnetic resonance imaging (MRI) is feasible for longitudinal monitoring of hepatic blood flow in patients with portal hypertension, before and after transjugular intrahepatic shunt (TIPS) implantation. It can provide an assessment of blood pressure changes induced by portal hypertension and has the potential of guiding treatment decisions regarding TIPS implantation. In addition, 4D flow MR imaging angiograms and blood flow measurements provide angiographic and quantitative evaluation of the hepatic hemodynamic response to TIPS implantation.
Our idea is to use contrast enhanced, 4D dynamic MRI sequences for the development and validation of a liver perfusion model that allows the evaluation of treatment efficacy for patients who have undergone chemoembolization therapy sequences.


