Using chitosan to combat osteoarthritis and aging cartilage - A complex sugar that can reduce inflammation
Montréal - Crustacean shells, particularly from shrimp, have held the fascination of many researchers because once transformed, they produce chitosan, a biopolymer with remarkable attributes that could make all the difference in the treatment of osteoarthritis.
In its pure form, chitosan consists of a linear chain of β-D-glucosamine linked to a variable number of N-acetyl-β-D-glucosamine units. This versatile polymer is what is also known as a complex sugar, an organic compound that is biodegradable and biocompatible, a biomaterial currently used in a variety of areas including agriculture and environment, with practical applications ranging from wastewater treatment to pharmacology. However, putting chitosan to work in the treatment of osteoarthritis has been a particular challenge. Caroline Hoemann, a professor in chemical engineering and biomedical engineering, and a biomaterials and tissue engineering specialist, wanted to remedy this situation by determining precisely how to use chitosan to preserve cartilage from degeneration.
Knees that click and crunch
Osteoarthritis affects a large percentage of the population and can arise when cartilage, a thin layer of tissue that covers the ends of bones, becomes worn away. Cartilage is an elastic and resilient tissue which acts as a shock absorber between the bones in the joint. In osteoarthritis, the cartilage tissue becomes worn to the bone. Osteoarthritis can also affect the spine, fingers, ankles and, most frequently, the knees. End result: chronic pain and reduced mobility.
“At this time, osteoarthritis treatments are symptomatic and mainly limited to treatments that aim to reduce joint pain. In this setting, macrophages are the bad guys. As immune cells that exist to protect the body, they can both protect or degrade the cartilage,” explains Professor Hoemann. “The solution we proposed is to use the chitosan to ‘guide’ the behaviour of the macrophages so that they produce mainly molecules that protect cartilage.”
Cartilage, a tissue unlike the others
Cartilage, a tissue that is attached to the ends of bones in the joint, is devoid of blood vessels. Instead, it bathes in a liquid that also acts as a lubricant. Within the thin tissue that produces this liquid, macrophages are found. They have a dual purpose: to produce molecules that nourish and protect the cartilage tissue, and others that protect the knee against an infection. Joint inflammation can develop. And when that inflammation becomes chronic, macrophages produce molecules that degrade cartilage.
To understand how to stimulate macrophages to sooth inflammation instead of aggravating it, Professor Hoemann’s team attacked the mechanisms of the inflammation itself. First, with her graduate student David Fong, they produced chitosan chains of varying length to see if this could make a difference. This intuition paid off.
Macrophages and phagosomes
When macrophages encounter chitosan, it is treated like a foreign body. First, the macrophages absorb chitosan into little pockets, called “phagosomes”, to analyze the material further. When the chitosan chain is the right length, it tricks the macrophages into sensing that they are faced with a bacterium. The result is that they produce anti-inflammatory molecules such as interferon-beta and the interleukin-1 receptor antagonist.
“If the chitosan chain is too long, the macrophages instead produce molecules that promote inflammation; if the chain is too short, nothing happens,” says Professor Hoemann. “The important discovery here is that a low dose of certain chitosans was identified as a new and promising way to counter inflammation and to protect the cartilage. This was not known until now.”
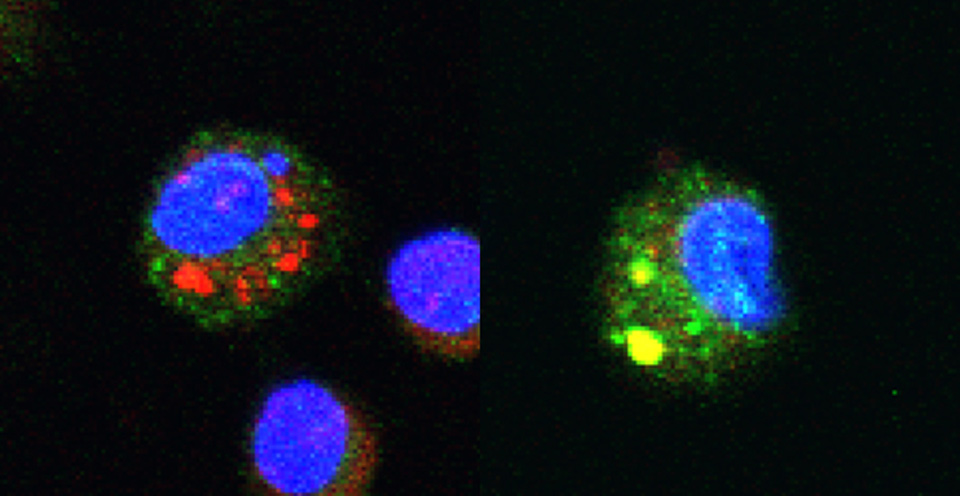
Left image: Chitosan, perceived as a foreign body, is absorbed and retained in the phagosomes of a macrophage (small red patches). Right image: the chitosan chain, when it is of the right length, becomes expelled from the phagosome (yellow patches); the macrophage reacts as though being faced with a bacterium and produces anti-inflammatory molecules.
A remarkable advance
By improving our understanding of the role of inflammation in tissue regeneration, Caroline Hoemann and her team have just made a breakthrough in uncharted territory that prevented chitosan from being the anti-osteoarthritis molecule that researchers had been investigating for many years. When it is the right chain length, chitosan stimulates the macrophages into releasing molecules that promote healing in situ, in the liquid that lubricates the cartilage surfaces.
Excellent results were obtained with in-vitro, as well as in-vivo, tests. Clinical testing in humans is envisioned in the near future. The ultimate goal? New therapies for people suffering from osteoarthritis, therapies that are available in the clinic.

From left to right: Caroline Hoemann, Professor in the Department of Chemical Engineering at Polytechnique Montréal; David Fong, PhD graduate in Biomedical Engineering at Polytechnique Montréal.
About Polytechnique Montréal
Founded in 1873, Polytechnique Montréal is one of Canada's leading engineering teaching and research institutions. It is the largest engineering university in Québec for the size of its graduate student body and the scope of its research activities. With over 45,700 graduates, Polytechnique Montréal has educated nearly one-quarter of the current members of the Ordre des ingénieurs du Québec. The institution offers more than 120 programs. Polytechnique has 250 professors and over 8,200 students. It has an annual operating budget of more than $210 million, including a research budget exceeding $70 million.


