Blogue
Biofabrication : contrôler la qualité des protéines… dès leur sortie de la cellule


By pairing a biosensor - like this one - with a bioreactor filled with cultured cells, Professor De Crescenzo wants to ensure that the quality of antibodies is monitored during the manufacturing process, rather than after its been completed. (Photo: Martin Primeau)
In his laboratory in the JA Bombardier building, Professor De Crescenzo's team is developing a strategy to measure the quality of biological drugs while they're being manufactured. It's an approach that's likely to reduce the costs of biofabrication processes, but will also guarantee patients reliable and effective drugs.
In recent decades, the pharmaceutical industry has accelerated the development of new therapeutic agents using cells themselves as manufacturing plants. In lieu of synthesizing small molecules through chemical reactions in glass balloons, cells are instead left to make peptides, proteins or viral particles capable of therapeutic or immunological action.
Other examples of this category of "biological drug" are vaccines to combat influenza or cervical cancer. Protein-based drugs, such as insulin or antibodies, are also included in this category. Professors Gregory De Crescenzo and Olivier Henry from Polytechnique Montreal's Department of Chemical Engineering are very interested in the latter sub-group, collaborating with Dr. Yves Durocher's team from the National Research Council Canada (NRCC) on the subject.
As is the case in the industry, the Polytechnique team uses bioreactors, which are a sort of incubator - in their case, they use 4 to 10 liter incubators. Inside, billions of cells are kept in suspension in order to produce and then secrete specific antibodies. Once purified and concentrated, these antibodies could theoretically be administered to patients for the treatment of various diseases such as cancer or rheumatoid arthritis, depending on the antibody type in question. However, this manufacturing process comes with its share of struggles, as was recently explained in this text from Labo 2500, featuring Professor Olivier Henry. In addition to maintaining uniform incubator temperature, researchers also need to keep an eye on pH and the availability of oxygen and nutrients - among many other variables. The stakes are high: cell productivity needs to be optimized, but so must the functionality and stability of the proteins manufactured.
“Despite every precaution, final product quality often varies from batch to batch - even if all the cultivation parameters have been respected. The problem is, you have to wait until the end of production to determine if everything has gone well," explains Professor De Crescenzo.
So clearly, it’s extremely important to come up with a solution that makes it possible to intervene in the process, in order to avoid discarding complete stocks of antibodies altogether.
At a glance... Therapeutic antibodies |
|
Antibodies are 800 to 1,000 times larger than chemically synthesized molecules. For example, if the active ingredient in aspirin (acetylsalicylic acid) weighed the same as a human, the antibody behind burosumab - seen above, in blue - would weigh the same as about 50 compact cars.
|
During rather than after
 Pr Gregory De Crescenzo (Photo : PolyPhoto)
|
With his team at Polytechnique Montreal, Professor De Crescenzo is currently working on a solution. The group's approach? Pairing a device that measures the ability of antibodies to bind to their therapeutic target - it's an indirect way of measuring their quality, and critically, while those antibodies are being made.
Antibodies are made up of long chains of small beads called "amino acids." Once assembled, these chains undergo a series of modifications in the cell. One such modification is the addition of sugars to the antibody surface - a crucial step in ensuring the quality of the final product. "Among other things, these sugars will influence antibodies' ability to bind to their target. Sometimes, without us really understanding why, they're not added correctly - and that influences the antibody's ability to do its job well," explains Professor De Crescenzo.
By relying on a surface plasmon resonance biosensor ("SPR"), his team wants to offer pharmaceutical companies a solution to analyzing the product while it is being manufactured. As a result, culture parameters could be adjusted to correct a possible problem, instead of simply notoing a problem once production is finished.
The Polytechnique Montréal team is currently testing its strategy in terms of the production of two antibodies, one used for the treatment of breast cancer (Herceptin / trastuzumab) and the other for the treatment of rheumatoid arthritis and certain forms of leukemia. (Retuximab).
AT A GLANCE... SPR biosensors |
|
Biodetection devices are not new, but the idea of pairing them with bioreactors is, in fact, rather new. The approach not only makes it possible to detect, but above all, to quantify the ability of a protein to attach to its target. To accomplish this, a gold foil is coated with billions of copies of the targeted protein. A droplet filled with the protein to be analyzed is then deposited on the surface of that same foil. In this example, it's an extract of the liquid contained in a bioreactor. By binding to the gold foil, the antibodies change the angle of refraction of the light under it, a characteristic identified by the device. The stronger the affinity the antibodies have for their target, the faster they'll stick to it, and the slower they will eventually be released. The device measures the kinetics of protein association and dissociation to determine the affinity of antibodies for their target. “Using this method, we immediately know whether the antibodies produced are functional,” explains Professor De Crescenzo. |
Learn more
Professeur Gregory De Crescenzo's expertise
Professeur Olivier Henry's expertise
Departement of chemical engineering website


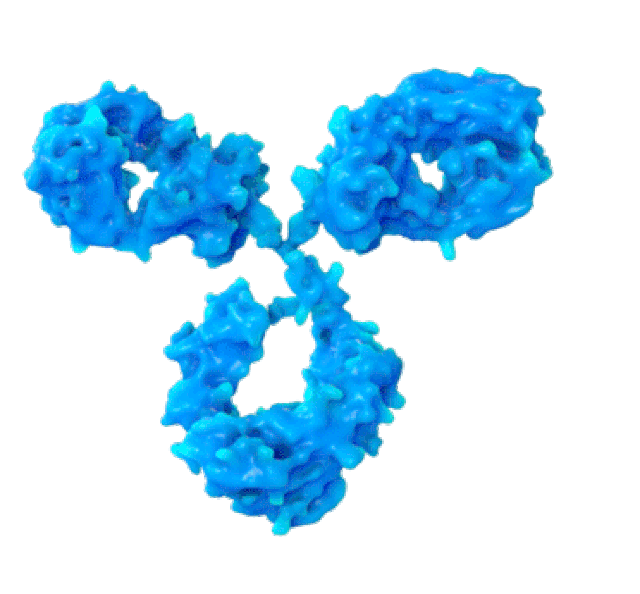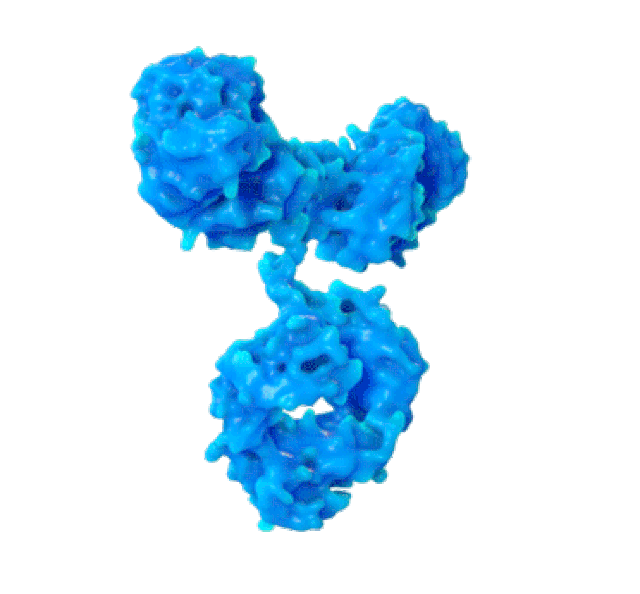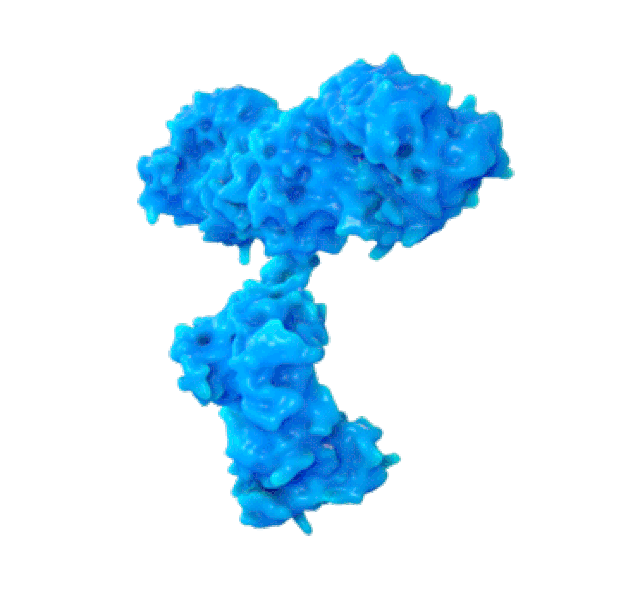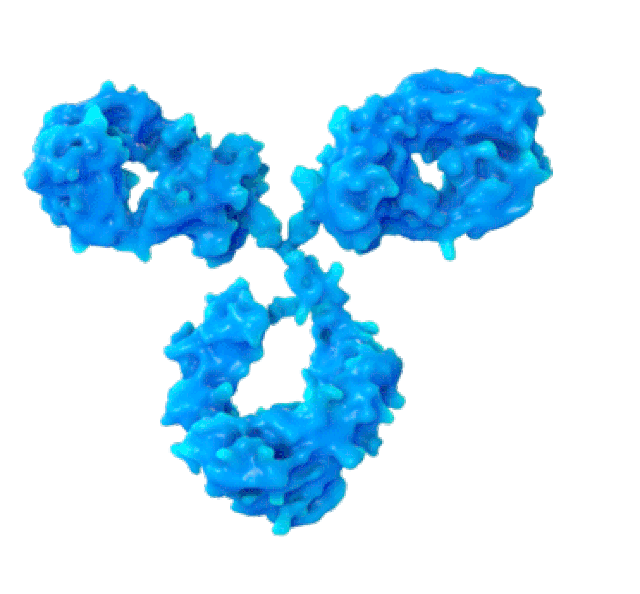
 SPR biosensors measure the speed with which antibodies associate with their target protein on this thin gold foil. (PHOTO: Martin Primeau)
SPR biosensors measure the speed with which antibodies associate with their target protein on this thin gold foil. (PHOTO: Martin Primeau)


Comments
Commenter
* champs obligatoire