Most fuels and niche chemicals derived from petroleum. Dwindling fossil resources and growing demand are pushing the chemical industry to focus on renewable resources.
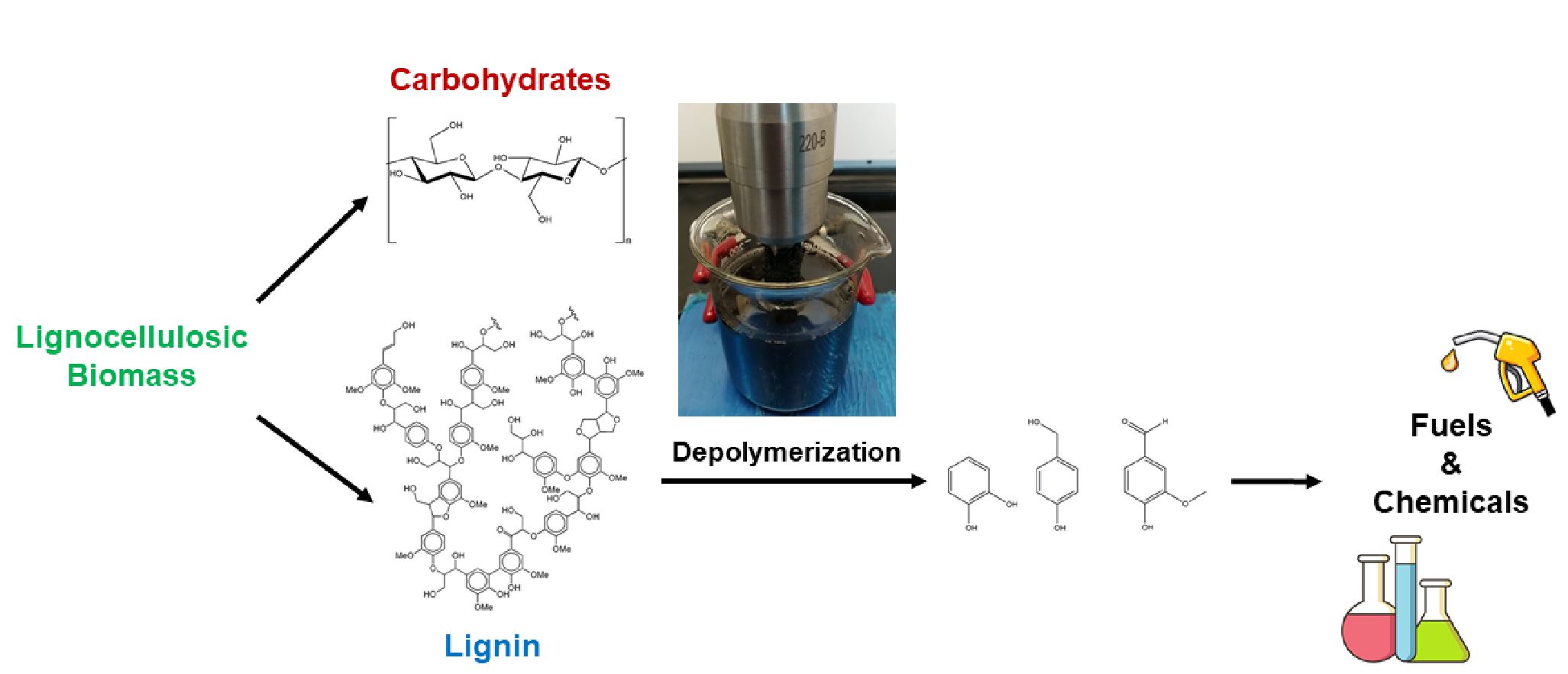
Lignocellulosic biomass is the most abundant clean raw material for the production of bio-based chemicals. The paper industry produces more than 50 million tonnes of lignin waste every year, most of which is burnt to power up the pulping plant. Lignin is the only carbon-rich biopolymer with an aromatic skeleton; it is an excellent platform compound for the green revolution that aims at originating a large number of value-added products, from plastics and paints to drugs and biofuels, with properties similar to those derived from petroleum. However, because of the complexity of its structure and the energy intensiveness of the depolymerization processes, less than 5% of the lignin separated from cellulose and hemi-cellulose is employed to manufacture valuable chemicals. In fact, the energetic cost of the current technologies cancels out any revenue augmenting from the sales of the products.
The EPIC team has expertise in intensified mechano-chemical sustainable biomass conversion. We focus on the depolymerization of lignin to polyphenols via ultrasound and microwave radiation and their conversion to biofuels, biolubricants, and other eco-friendly useful chemicals.
Low-frequency ultrasound (US, 20 kHz to 120 kHz) finds applications in many fields as a process intensification technology. When combined with heterogeneous catalysis, US promotes and accelerates reactions, and increases the yield of organic syntheses through cavitation.
Acoustic cavitation is the formation, growth, and implosive collapse of bubbles in a liquid irradiated with ultrasound. When sound waves pass through the liquid, they generate areas of compression (positive pressure) and expansion (negative pressure). At very low pressure in the expanding region, the intermolecular spaces exceed the critical molecular distance, and voids or microbubbles form in the liquid. The size of these vapor-filled cavities oscillates in phase with the compression and expansion cycles (stable cavitation), thus growing under the effect of the sound pressure field. Over few cycles, they grow and reach an unstable size and implode violently, releasing phenomenal energy over a very short period of time: the pressure and the temperature reach locally about 1000 MPa and 3273 K, respectively. At these conditions, the molecules trapped in the cavitating bubbles undergo pyrolysis reactions generating highly reactive radicals. In addition, cavitation induces local turbulence and microcirculation of the liquid thereby improving heat and mass transfer. The combination of the chemical and physical effects of cavitation initiate and propagates chemical reactions.
In our group, we are investigating the chemical effects of acoustic cavitation by characterizing and quantifying the free radicals generated during the sonolysis of some organic and aqueous solutions in the presence of solid particles. Electron paramagnetic resonance (EPR) is able to quantify the alkyl (R •), hydroxyl (• OH), and alkoxy (• OR) radicals. We aim to identify the optimal conditions (solvent with the best acoustic activity, temperature, diameter and concentration of the solid particles, ultrasound power) to maximize the production of radical species in the reaction medium and intensify the heterogeneous reaction associated with ultrasound.
The application of ultrasound has absorbed a lot of attention in recent years due to its chemical and physical effects. Ultrasound power has been used for mixing, emulsification, the formation of radicals and oxidizing species, ultrasonic desulfurization, homogenization, cell disruption, and extraction. The basic underlying physical phenomenon behind all the mentioned applications is acoustic cavitation. The propagation of the ultrasound wave changes the pressure within the system. The distribution of the acoustic pressure in sonicated liquids is non-uniform, and the formation of acoustic cavitation depends on the local sound pressure. When the acoustic pressure exceeds the threshold pressure value of inertial cavitation, bubbles violently collapse and produce extremely high pressure and temperature.
To keep these phenomena under control in various applications, we first need to follow the evolution of physical parameters (pressure, temperature, composition) of the gas inside the cavitation bubbles and the liquid outside since the two are linked one to another. Second, for most practical sonochemical reactions, we need to specify the factors that affect acoustic cavitation. The right selection and optimization of these factors help us to apply proper energy intensity and, as a result, have higher energy efficiency. In sonoreactors, cavitation occurs in the forms of clusters and clouds. Thus, it is the dynamics of the bubbles in these clusters that determine the cavitation related phenomena. Due to the complex interaction between cavitation bubbles in a cluster and the uncertainties in the spatial distribution of bubble size and bubble density.
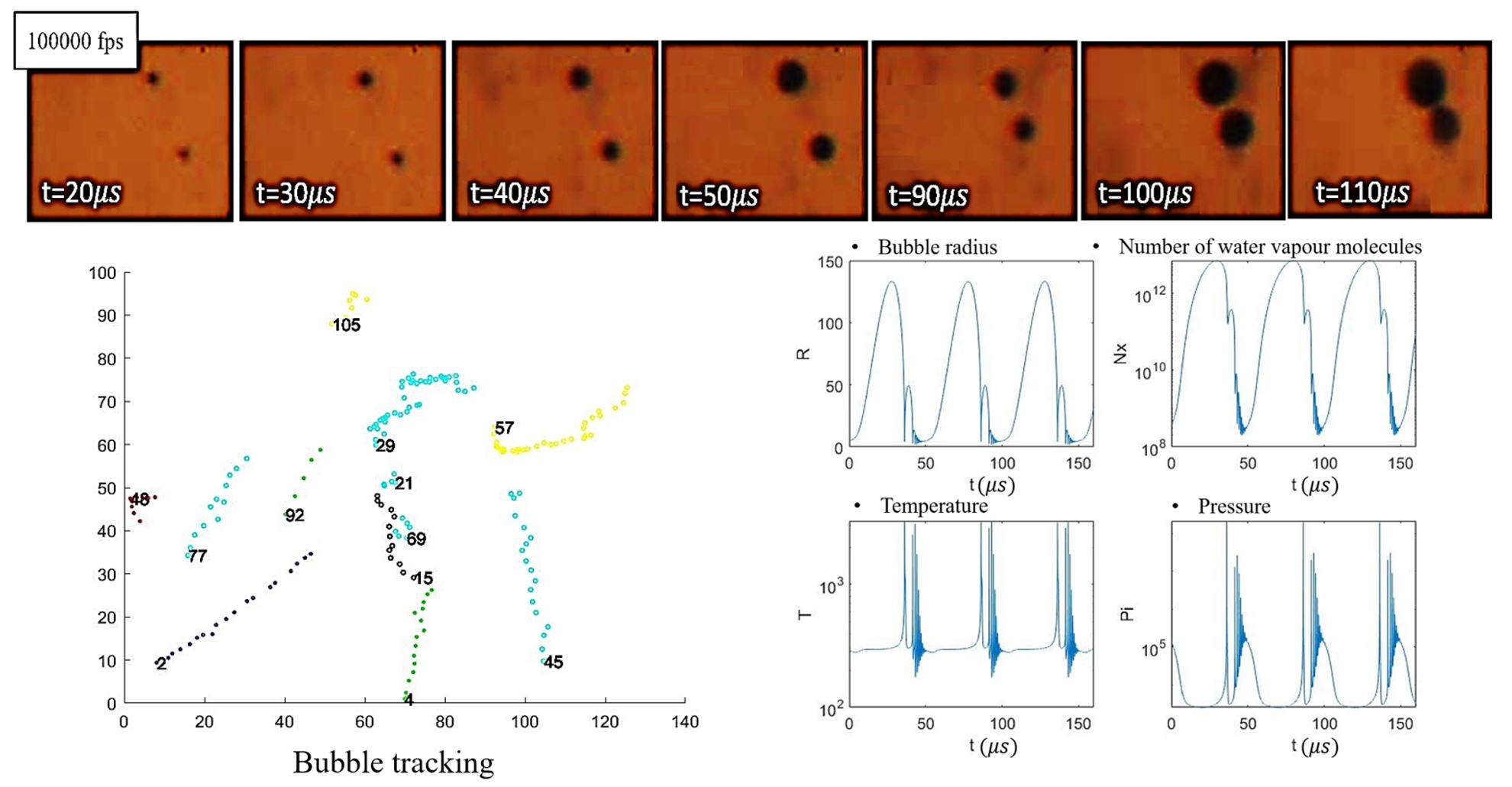
In our group, we predicted the bubbles' behavior in the acoustic field that appears in clouds of bubbles, with the combination of experimental and numerical techniques. We capture the oscillation of transient cavitation bubbles in a cluster with the Schlieren technique and an ultra-high-speed camera connected to the long-distance microscope. We can track cavitation bubbles and obtain their oscillation as a function of time and space. We apply mathematical modeling to investigate the behavior of strongly collapsing bubbles under ultrasound irradiation.
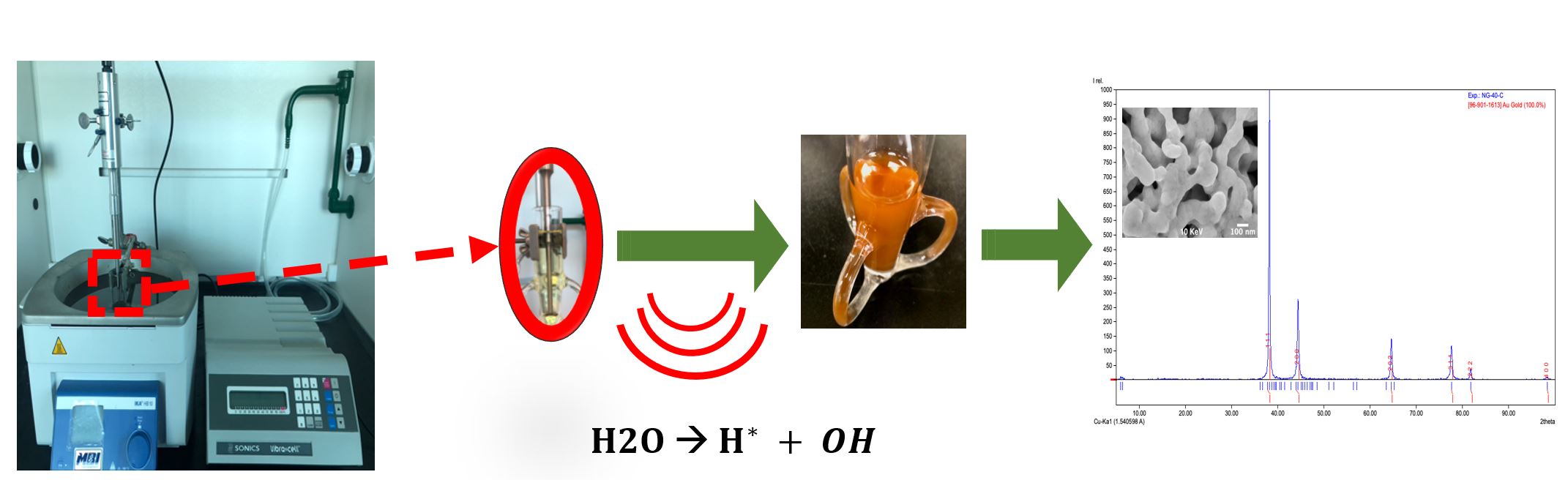
The combination of nanotechnology and medicine has advanced the use of gold nanoparticles (AuNPs) as promising candidates for the design of novel drug delivery systems and the investigation of biological singularities. Physical and chemical properties of AuNPs, such as size, shape, chemical composition, and surface area (both in the macroscopic and microscopic or nanoparticulate states) generally influence their application.
The obtainment of highly desired structures (rod, sphere, and cages) may be achieved through the variation or combination of reducing or capping agents. However, conventional synthesis methods require high temperatures, toxic capping agents (eg CTAB), and long synthesis times to produce novel 1 or 2-dimensional structures. Ultrasound irradiation has proven to be a suitable process to synthesize nanomaterials without the use of hazardous substances while increasing reaction rates and yields. The physical and chemical effects of ultrasound provide a wide range for nanomaterial formation of various size and shape when compared to conventional routes.
In our group, we use green synthesis and process intensification through the combination of ultrasound irradiation with biocompatible molecules to synthesize porous, crystalline AuNPs with controlled sizes suitable for drug delivery applications.
Lactic acid is a naturally occurring hydroxycarboxylic acid in the human body and can be synthesized via fermentation or chemical synthesis. It spans the food, pharmaceutical, and cosmetic industries. In addition, the production of lactic acid from renewable feedstocks is gaining traction. Lactic acid's appeal stems from its polymeric form - polylactic acid (PLA). PLA is a prized molecule for biomedical applications - sutures, implants, scaffolds, targeted drug delivery - thanks to its biodegradability and biological compatibility. It degrades through the hydrolysis of its ester groups and is harmlessly expelled or consumed by the body through the tricarboxylic acid cycle, in low concentrations.
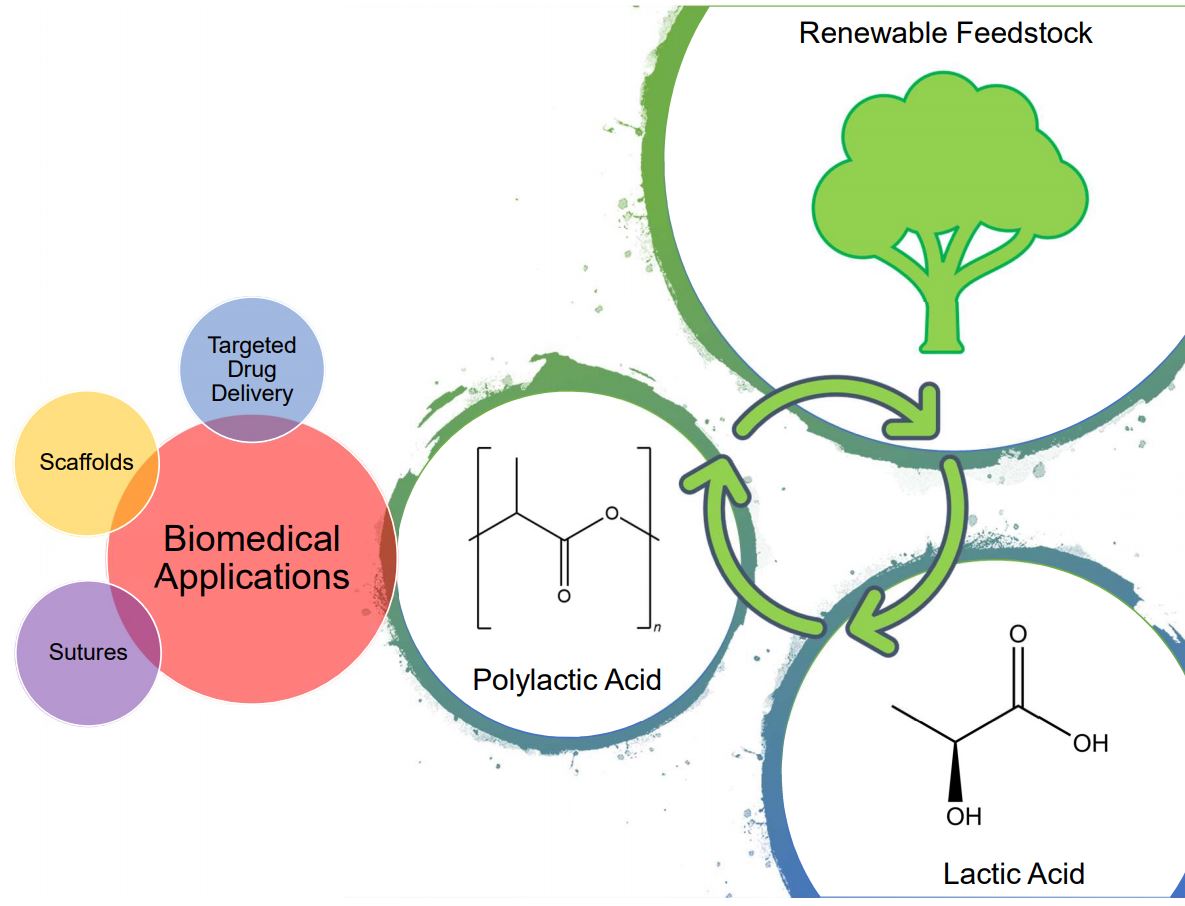
Dehydration of lactic acid yields lactide, which can be further polymerized to PLA. The two common methods are polycondensation and ring-opening polymerization. The latter being the more favorable. Various catalysts drive this reaction - organic, metallic, and enzymatic. However, current manufacturing methods are laborious, lacking, and often introduce impurities in the PLA hindering its efficacity. We are striving for an efficient process rooted in microwaves. In our research group, we are tackling this challenge by exploring different production avenues with catalysts, solvents, and microwave settings.
When the catalyst and reagents are in more than one phase (heterogeneous catalysis), the chemical reaction is limited to the phase boundary as it is the only place where both reactants and catalyst are present. The specific surface area of the interface plays an important role in the reaction rate. Moreover, the products of the chemical reaction accumulate at the interface thus preventing other molecules from reacting.
Ultrasound effectively disperses solids in liquid media and emulsifies immiscible liquids. The reduction of the droplet size of the dispersed phase increases the phase boundary surface area, which in turn increases the rate of the chemical reaction. Also, the mechanical shear forces caused by cavitation causes turbulent flow and promotes mass transfer.
In our group, we apply ultrasound to tune the properties (eg band-gap and crystallinity) of catalysts during synthesis, and to intensify heterogeneously catalyzed organic reactions (eg esterification, transesterification, and epoxidation).
Photocatalysis is an advanced oxidative process with a variety of environmental applications including water and air treatment. The strong oxidative potential of this technique makes it ideal for degrading recalcitrant pollutants that pose a threat to both human and environmental health. Photocatalysts (eg semiconductors) are capable of harnessing photons and initiating chain reactions by producing reactive oxidizing species or reacting directly with pollutants. Historically, TiO 2is the most used photocatalyst but with limitations in efficiency and the requirement of UV-radiation for photoexcitation. Alternative semiconductors are being used and developed. The modification and design of new photocatalysts offer a unique opportunity to alter the composition and physicochemical properties to optimize catalyst parameters and increase activity and degradation efficiency.

The EPIC group is involved with the synthesis of novel catalysts using a variety of methods including sol-gel, spray drying, impregnation, ultrasound, and micronization. We also focus on characterizing these catalysts and evaluating their photocatalytic activity to identify candidates for industrial application. Above all, our focus is on designing micro-sized catalyst vs. nano-sized ones to eliminate the health concerns around the adoption of nanoparticles.
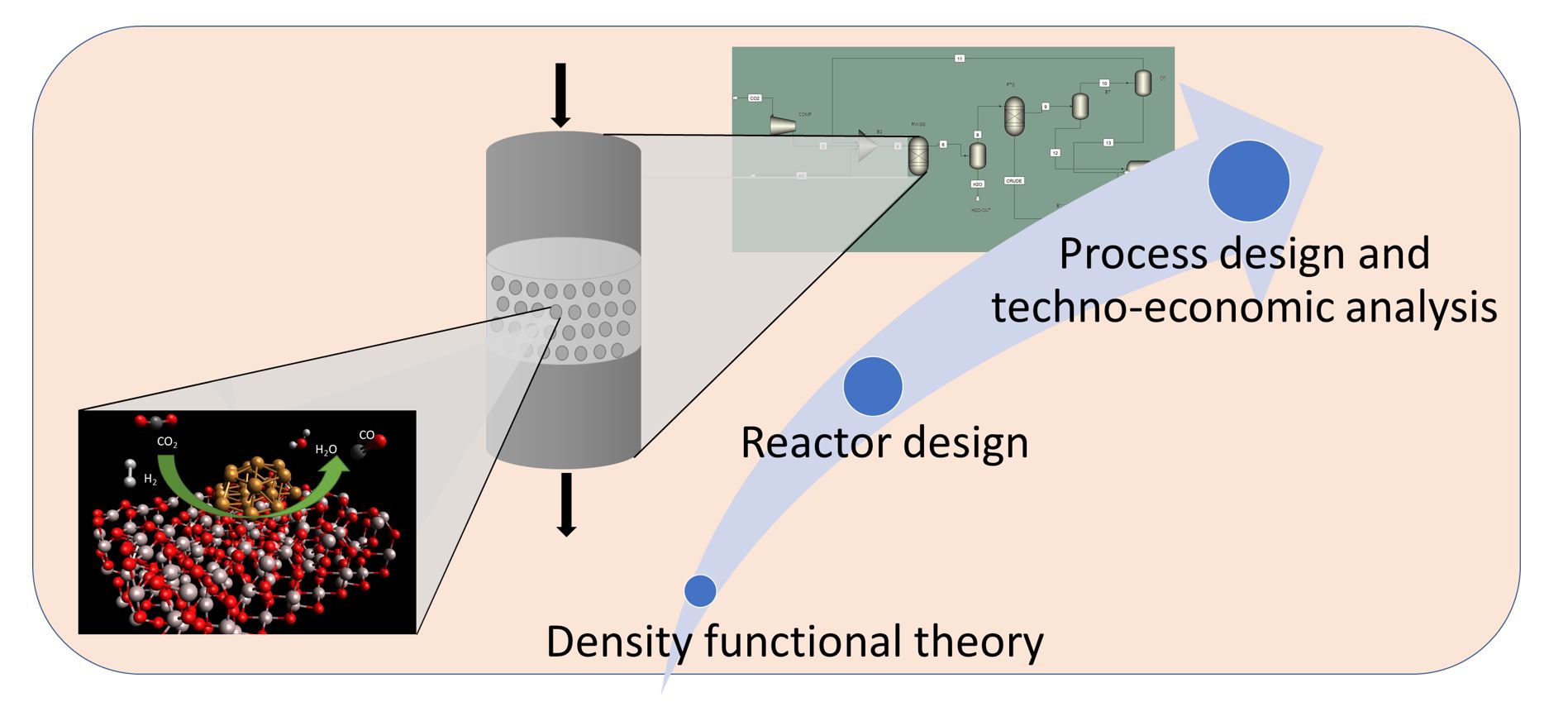
The EPIC group fosters the transition towards sustainable jet fuel by following a multi-scale approach.
We tackle all the aspects of gas-to-liquids (GtL) and power-to-liquid (PtL) technologies. They involve two catalytic reactions, namely the production of syngas (via methane partial oxidation or CO2 reverse water gas shift), and the Fischer-Tropsch process. First, we evaluate the reaction mechanism at the atomic scale with density functional theory to define the optimal catalyst compositions for each reaction. Understanding the reaction mechanism is key to select the optimal reaction conditions and design the reactors. We validate the computational results experimentally. Finally, we also tackle the techno-economic analysis of the entire process to evaluate the economic feasibility of the designed process.
Coming Soon
Metal-Organic Frameworks, as a subset of Coordination Polymers (CPS) with potential voids, have become a highly competing porous material with zeolites and metal oxides. The great expansion in this class has brought various structures with remarkable chemical, physical, textural, and thermal properties. However, there is still more to discover. Deep comprehension of how MOFs interact can help to control the formation of targeted structures, and the connections within complex networks.
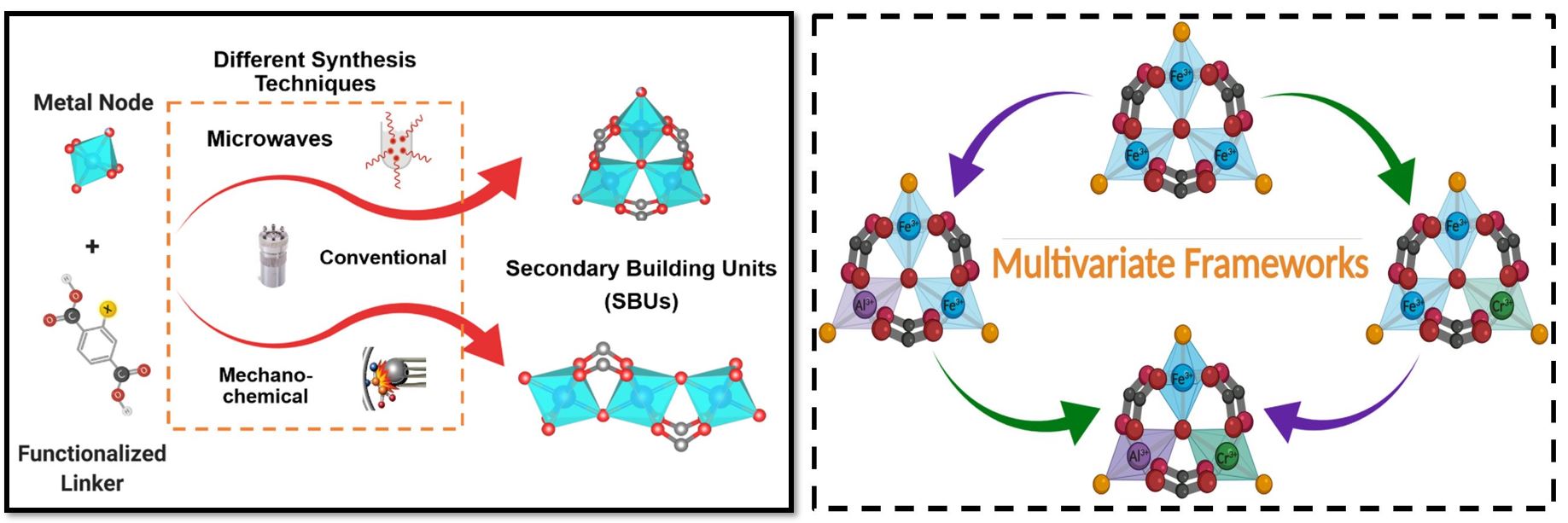
 In the EPIC lab, we focus on the chemistry behind these materials on both the micro and the macro scales. With the aid of the available assorted utilities, we investigate MOF synthesis via microwaves, ultrasonic waves, mechano-chemical techniques, besides the conventional hydrothermal/solvothermal method. Introducing flexibility to robust frameworks, multivariate structures, framework functionalization, and nanoparticle decoration, are the main themes of our research. Our aim is to explore and extend the applications of these interesting materials in gas adsorption and separation, catalysis, drug delivery, and sensing.
In the EPIC lab, we focus on the chemistry behind these materials on both the micro and the macro scales. With the aid of the available assorted utilities, we investigate MOF synthesis via microwaves, ultrasonic waves, mechano-chemical techniques, besides the conventional hydrothermal/solvothermal method. Introducing flexibility to robust frameworks, multivariate structures, framework functionalization, and nanoparticle decoration, are the main themes of our research. Our aim is to explore and extend the applications of these interesting materials in gas adsorption and separation, catalysis, drug delivery, and sensing.
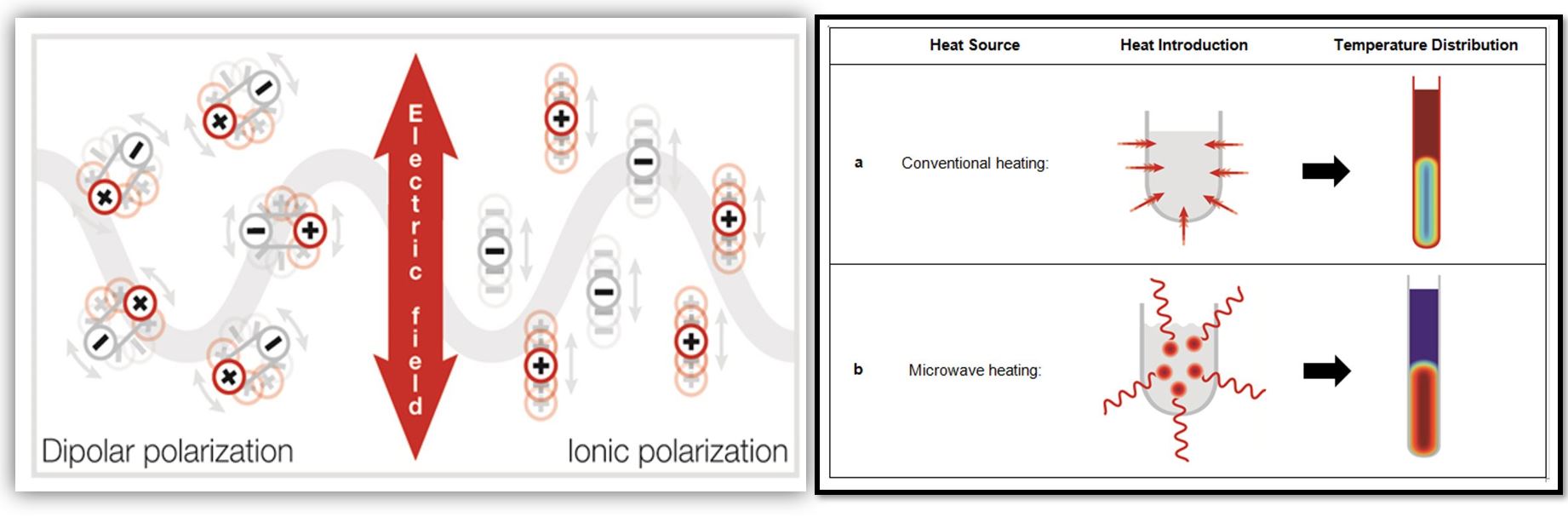
Microwaves, as a high-frequency electric field, have been involved in chemical engineering to promote chemical reactions at elevated temperature is a promising technique in terms of saving energy requirements of a variety of processes. In a typical microwave reaction, heating takes place by the dielectric heating effects, whereby the sample interacts with the generated electromagnetic field based on two major mechanisms: dipolar polarization and ionic conduction. The ongoing research success of the microwave-assisted synthesis has resulted in increased utilization of this approach. This approach enabled us to achieve higher reaction temperatures (superheating of the solvents), decrease the energy consumption and the reaction time (by the direct “in-core” heating), and obtaining higher yields and purer compounds (minimizing wall effects and faster heating rates). Moreover, the heat production in microwave systems is homogenous compared to the conventional heating methods as the reaction medium itself is internally heated by its components. With many ongoing projects utilizing microwaves, we are expanding our scope to involve its application in various engineering fields.
There are a plethora of valuable functional molecules waiting to be harvested from food waste. The benefits are two-fold: alleviating environmental strain by reducing this waste and capitalizing on the utility of these molecules. For example, citrus fruit peels are home to pectin, a family of polysaccharides composed mainly of covalently bonded galacturonic acids. It is common in the food industry to make jams, fillings, sorbets, and so forth because of its ability to form gels. Pectin is also a promising molecule for human health; it decreases cholesterol levels, it is immunostimulating, potentially anti-cancerous, and has drug-delivering capabilities.
Commercial pectin extraction requires high heat (> 60 oC), long reaction times (up to 6 h), and a low pH (below 3). Our research group is centered around process intensification (PI); we want to maximize yield in a minimum time. Our approach revolved around ultrasound and microwave extraction, two technologies that enable our reach for PI.
This approach can be clearly applied to several functional molecules present in different matrices.


